The heat treatment process is generally composed of three stages of heating, heat preservation and cooling. The purpose is to change the internal structure of the metal and alloy so that the material meets the performance requirements of the service conditions.
In order to obtain the required microstructure and properties of the steel after heat treatment, most heat treatment processes (such as quenching, normalizing and ordinary annealing) need to heat the steel to the critical point A1 or A3, see Figure 2-1. Forms an austenitic structure called austenitization and then cools it in a certain way (or speed). Therefore, the transformation of steel during heating is the basis of steel heat treatment, and the microstructure and properties of the heat-treated steel are largely related to the austenite phase formed upon heating. If the overheating will cause austenite grain growth, the impact toughness of the steel after heat treatment will decrease, showing a clear embrittlement tendency. It is important to study the heating transformation to improve the heat treatment process of steel. Mastering the heating transformation law is the theoretical basis for learning various cooling phase transitions. This chapter will focus on the formation of austenite during equilibrium heating.
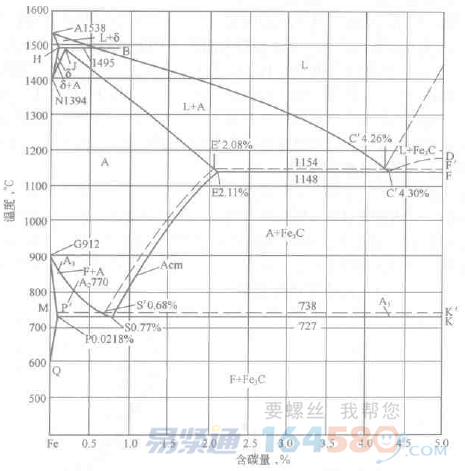
Figure 2-1 Fe-Fe3C phase diagram (solid line) and Fe-graphite phase diagram (dashed line)
1 Overview
During the actual heat treatment of the heat treatment, a non-equilibrium phase transition often occurs and cannot be completely analyzed by the Fe-Fe3C phase diagram. Therefore, in order to grasp the formation law of austenite, it is necessary to study the thermodynamic conditions, formation mechanism, dynamics and influencing factors of austenite formation.
According to the Fe-Fe3C phase diagram (see Figure 2-1), when the temperature is below Al, the equilibrium structure of the eutectoid carbon steel is pearlite, the meta-eutectoid carbon steel is pearlite plus ferrite, and the hypereutectoid carbon steel is Pearlite plus cementite. The pearlite structure is a mechanical mixture composed of ferrite and cementite. Therefore, in terms of phase composition, the equilibrium phase of carbon steel below Al temperature is iron and cementite. When the temperature exceeds Al, the pearlite will transform into single-phase austenite. As the temperature continues to rise, the pro-eutectoid ferrite in the hypo-eutectoid steel will be transformed into austenite, and the pro-eutectoid cementite of the hypereutectoid carbon steel dissolves into the austenite, causing the austenite amount to gradually increase. The chemical composition of austenite varies along the A3 and Acm lines, respectively. When the heating temperature exceeds the GSE line, the equilibrium phase is single-phase austenite.
It is known from thermodynamics that the power of phase transformation when steel is heated is the difference in volume free energy between the austenite and the parent phase of the new phase V·Δgv. When austenite nucleation, the free energy of the system changes: △G =V·△gv ten Sσ+εV (2-1)
Sσ—the interfacial energy added when austenite is formed;
εV—the strain energy that is added when austenite is formed.
Since the austenite is formed at a high temperature, the strain energy of the phase change is small, so the resistance of the phase change is mainly the interfacial energy.
Figure 2-2 shows the free energy of the eutectoid steel austenite and pearlite as a function of temperature, the intersection point is A1 point (727 ° C). When the temperature is equal to 727 ° C, the pearlite and austenite free energy are equal, and the phase transition does not occur. When the temperature is higher than A1, Δgv is a negative value, that is, the first term on the right side of the formula (2-1) is a negative value, and then a phase change may occur. V·Δgv is the driving force for austenite formation, which increases as the heating temperature increases. Only above the A1 point, when the driving force V·Δgv of pearlite transformation to austenite can overcome the increased interfacial energy and strain energy of austenite formation, austenite will spontaneously form, ie austenite Formation must have a certain degree of superheat (△T).
Therefore, when heating and cooling, the phase change does not proceed at the temperature shown in the phase diagram, usually under a certain superheat or supercooling. The degree of superheat or undercooling increases as the heating rate or cooling rate increases. Thus, the critical point at the time of heating and cooling is not at the same temperature. Usually, the critical point of heating is marked with the letter c, such as Ac1, Ac3, Accm, etc.; and the critical point of cooling is marked with the letter r, such as Ar1, Ar2, Arcm, and the like. Figures 2-3 show the movement of the critical point at both the heating rate and the cooling rate of 0.125 ° C / min.
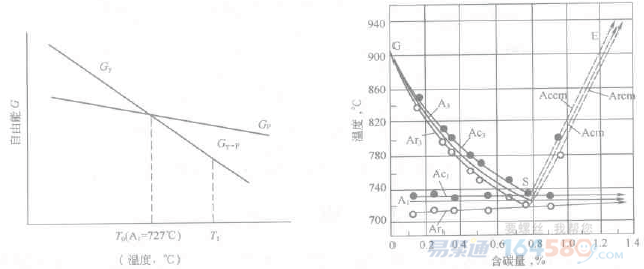 Figure 2-2 Relationship between pearlite (P) and austenite (γ) free energy and temperature Figure 2-3 Movement of critical point in Fe-Fe3C phase diagram
Figure 2-2 Relationship between pearlite (P) and austenite (γ) free energy and temperature Figure 2-3 Movement of critical point in Fe-Fe3C phase diagram2. Austenite organization, structure and properties
Austenitic structures are usually composed of equiaxed polygonal grains, and twins are often observed in the crystal, see Figure 2-4. Austenite is a solid solution of C in γ-Fe. The C atom is at the center of the octahedral gap composed of Fe atoms in the γ-Fe lattice, that is, the center of the face-centered cubic unit or the midpoint of the edge, as shown in Figs. If all the octahedral gap positions are filled with C atoms, the unit cell contains 4 Fe atoms and 4 C atoms, that is, the atomic concentration is 50%, and the equivalent mass concentration is 20%, but actually Austen The maximum carbon content of the body is 2.11% (mass), and the atomic concentration is 10%, that is, there are 10 C atoms in the 25 γ-Fe unit cells. This is because the radius of the C atom is 0.77A, and the radius of the octahedral gap in the γ-Fe lattice is only 0.52A. Therefore, when the C atom enters the gap position, the lattice distortion is caused, so that the surrounding space is not May fill up the C atom. In fact, C is statistically evenly distributed in austenite, and there is a concentration fluctuation.
 Figure 2-4 Austenite in steel (500×) Figure 2-5 Possible gap position of C in γ-Fe
Figure 2-4 Austenite in steel (500×) Figure 2-5 Possible gap position of C in γ-FeThe presence of C atoms causes the austenite lattice to expand, so the lattice constant increases as the carbon content increases, as shown in Figure 2-6.
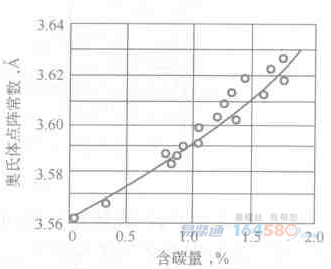
Figure 2-6 Relationship between austenite lattice constant and carbon content
Austenite in alloy steel is a solid solution in which C and alloying elements are dissolved in γ-Fe. Alloying elements such as Mn, Si, Cr, Ni, C. Or, a substitutional solid solution is formed by substituting the position of the Fe atom in γ-Fe. Their presence also causes lattice distortion and lattice constant changes. Therefore, the lattice constant of the alloy austenite is related to the content of the alloy element and the difference between the alloy element atom and the Fe atom radius.
Among the various microstructures of steel, austenite has the smallest specific volume, the largest coefficient of linear expansion, poor thermal conductivity, high plasticity, low yield strength, and easy deformation processing. For steels containing 0.8% C, austenite, and ferrite The specific volume of the body and martensite are 0.12399cm^3/g, 0.12708cm^3/g and 0.12915cm^3/g, respectively, and the linear expansion coefficients are 23×10-6cm/K and 14.5x10-6cm/K, respectively. And 11.5 x 10-6 cm/K. In the industry, gauge elements are often produced using a large linear expansion coefficient of austenitic steel. In carbon steel, the thermal conductivity of ferrite, pearlite, martensite, austenite and cementite are 77.1W/(m·K), 5l.9W/(m·K), 29.3W/ (m·K), 14.6 W/(m·K), and 4.2 W/(m·K). In addition to carburizing, austenite has the worst thermal conductivity. Therefore, when austenitic steel is heated, it is not advisable to use an excessive heating speed to avoid deformation of the workpiece due to excessive thermal stress.
Silicone Roof Flashing,Roof Flashing,Tpr Rubber Pipe Boot,Custom Lead Flashing
Ningbo Yongsheng Rubber And Plastic Products Co., Ltd. , https://www.yscnrubber.com